


Immundiagnostik has been implementing the European Regulation on In Vitro Diagnostics (IVDR 2017/746/EU). Our company continues to ensure the current and future availability of our products under this regulation.
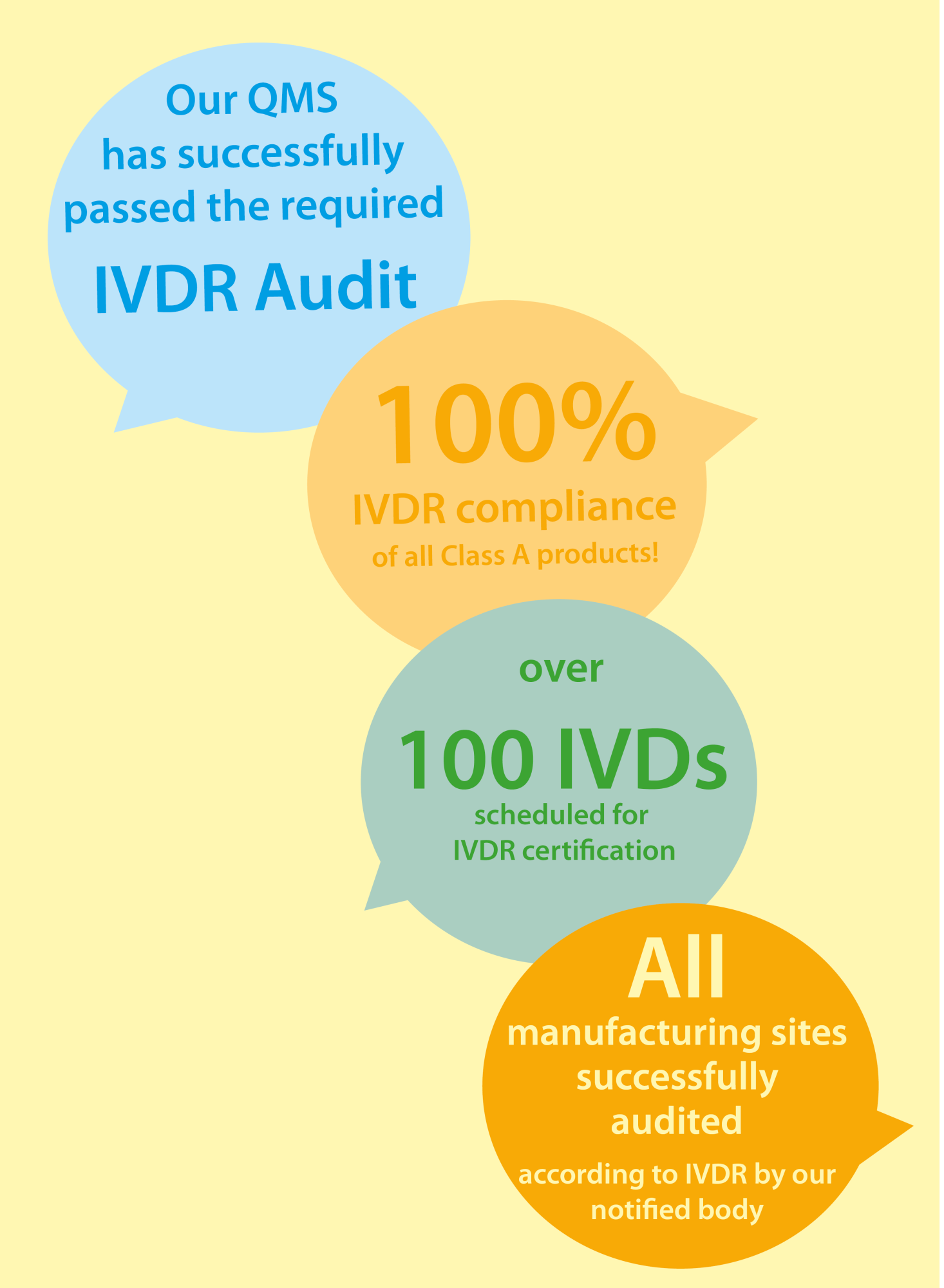
We will continue to keep you informed about the latest developments!